Revolutionizing Clinical Trials with EasyCLIN®
Launching EasyCLIN® an advanced clinical trial management software (CTMS) suite with a built-in database, ensuring efficiency, compliance, and cybersecurity.
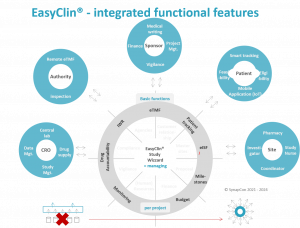
Our EasyCLIN® suite simplifies the clinical trial process with user-friendly tools and reliable technology, offering unmatched data integrity, efficiency and precision.
Components of the EasyCLIN® offering:
· EasyFeasibility - a portal that allows sponsors and CROs to determine the suitability of potential study sites for a clinical trial.
· EasyTMF® - an electronic trial master file that incorporates real-time user and project management, allowing a sponsor to track the components of a trial and have documents and version history ready for regulatory or internal reviews.
· EasyISF® (investigator study file) - Site specific clinical trial relevant documentation management.
· Full technical, regulatory and compliance support, customization availability, and data storage options.
Key Advantages:
· Comprehensive Trial Management: EasyCLIN® compiles all information for the final processing and reporting of a trial, providing an easy-to use nucleus and end-to-end coverage for any clinical research project.
· Regulatory Compliance: The software suite is designed to comply with global regulatory standards, including FDA 21 CFR Part 11. In particular, it is designed to be EU GDPR compliant, making it easier for a sponsor to expand into European markets, and any region with strong data privacy protections.
· Study Site Collaboration: Individual study sites can also use the system to fill out questionnaires and provide other information related to the trial, and EasyTMF® allows sponsors to easily track when requirements are met and stages are completed.
· Built-in Database: EasyCLIN® offers its own built-in SQL database, providing easy document and data access.
· Efficient and Sustainable Research: EasyCLIN® is designed to help clients move off paper-based systems and execute fully electronic clinical trial document management, reducing both the time and the waste needed to conduct a trial.
About CyberActa, Inc.
A professional services provider for heavily regulated industries and sectors, headquartered in Boston, Massachusetts, offering global data-driven digital, regulatory, cyber, and privacy solutions. The company's products and services are used by Fortune 500 companies, government agencies, and startups around the world. Maria Papademetris, Corporate Development Engineer, is assuming the SynapCon portfolio management in the United States.
About SynapCon GmbH.
A technology-driven medical research company. Using the latest scientific findings and technologies to open-up new therapeutic and diagnostic fields and to bring life-prolonging and life-saving medical products to market faster and safer. SynapCon works on their own projects as well as those for the research industry, hospitals and other organizations.
Maria Papademetris - Business Development/SynapCon Portfolio
CyberActa, Inc.
+1 617-830-3041
email us here
Visit us on social media:
X
LinkedIn
Distribution channels: Healthcare & Pharmaceuticals Industry, IT Industry, Manufacturing, Shipping, Storage & Logistics, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release